
| World Plastics News |
MedShape's Novel Orthopedic Implants Are Made of Solvay’s PEEK resin
March 19, 2013 – MedShape Inc., a leading supplier of orthopedic implants featuring patented shape memory technology, has designed and manufactured the Morphix® Suture Anchor for tendon and ligament repair and the ExoShape® Soft Tissue Fastener for knee joint ACL reconstruction using Zeniva® polyetheretherketone (PEEK) resin from Solvay Specialty Polymers, which is part of Solvay’s line of Solviva® Biomaterials. MedShape is the first to develop and commercialize FDA-cleared devices manufactured from shape memory polymers. Using Zeniva® PEEK as the base material, MedShape has developed a proprietary shape memory PEEK called Altera® which allows devices to enter the target surgical site in a compact geometry and then be triggered to deploy with minimal mechanical force into the optimal geometry for fixation.
According to MedShape, active rehabilitation can cause anchor migration and loosening which may lead to clinical failure of the repair. Laboratory testing has shown that traditional anchor pullout can occur below 1,000 cycles at a load less than 50% of initial pullout strength. The Morphix® Suture Anchor responds positively to cyclic loading due to its dynamic geometry and stored shape memory strain. After implantation, cyclic loading stimulates the Morphix® Suture Anchor to attain its permanent, fully open “zero-strain” state. This results in continued wing expansion and retention of initial pullout strength, according to the company. The shape memory Morphix® Suture Anchor is delivered pre-compressed in a low-profile geometry that inserts easily into the surgical site, utilizing a simple and reproducible tap-in technique. It is available in 2.5-mm, 3.5-mm, 4.5-mm, and 5.5-mm diameters and a range of suture and needle configurations.
The ExoShape® Soft Tissue Fastener provides a straightforward, non-rotational insertion and expansion which eliminates “graft wrap” and preserves the preferred graft orientation. The graft bundles stay exactly where they’re placed, promoting a more anatomic reconstruction, according to Kathryn Smith, MedShape marketing manager. Other fixation devices can drive the graft back up the tibial tunnel, introducing unwanted graft laxity. The ExoShape® Soft Tissue Fastener’s “closed force loop” design eliminates this problem by preventing retrograde force being applied to the graft, according to the company. Zeniva® PEEK is a comparable or better-performing alternative to metals such as titanium for implantable devices, according to Solvay. The material offers many important benefits including biocompatibility, chemical inertness, and a modulus of elasticity that is close to that of bone. Based on biocompatibility testing, Zeniva® PEEK demonstrates no evidence of cytotoxicity, sensitization, irritation, or acute systemic toxicity, and meets the ASTM F2026 standard. It also boasts high strength and stiffness and has radiolucent properties which enable x-ray procedures without interference. “We’ve been very pleased to work with MedShape as they develop improved approaches for orthopedic fixation devices,” said Shawn Shorrock, global healthcare market manager for Solvay Specialty Polymers. “The ongoing acceptance of Zeniva® PEEK has validated our approach to the orthopedic implant market and we’re encouraged by the momentum we’ve generated.” The manufacturing site for Zeniva® PEEK and other Solviva® Biomaterials in Alpharetta, Ga., is ISO 13485 registered and the relevant aspects of current Good Manufacturing Practices are also applied. Solvay’s biomaterial manufacturing processes are carefully validated and enhanced controls provide product traceability. In addition, all materials are tested in an ISO 17025 accredited lab. In addition to Zeniva® PEEK, Solvay’s Solviva® Biomaterials line includes Proniva® self-reinforced polyphenylene (SRP), one of the world’s stiffest and strongest unreinforced thermoplastics that offers exceptional chemical resistance and hardness; Veriva® polyphenylsulfone (PPSU), which provides unsurpassed toughness combined with transparency and excellent chemical resistance; and Eviva® polysulfone (PSU), which offers practical toughness in a strong, transparent polymer. These sterilizable products are available in injection molding and extrusion grades as well as rods and plates for machined components. MedShape Inc. is a privately held medical device company working to develop and commercialize a portfolio of surgical solutions that use its patented shape memory technologies to address the increasing demand for improved sports medicine, joint fusion, and musculoskeletal trauma products. Solvay Specialty Polymers is a leading global supplier of high-performance thermoplastics for implantable and non-implantable medical devices. The company has expanded its focus on the healthcare industry to meet the growing needs of its global customers. Solvay is building on its 20-year history as a key material supplier in the healthcare field, devoting considerable new resources to help customers be more efficient and cut costs. Metal-to-plastic replacement remains a key focus for manufacturers, but increased cost pressures pose a new challenge as the market continues to grow at a double-digit pace. Solvay also continues to devote considerable research and development activities to polymer technology and commercialization of new and unique material options for medical OEMs and processors. Source : Solvay |
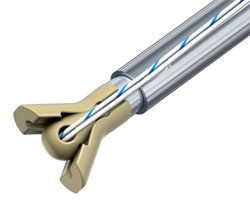 Suture Anchor and Soft Tissue Fastener Feature Unique Shape Memory Technology
Suture Anchor and Soft Tissue Fastener Feature Unique Shape Memory Technology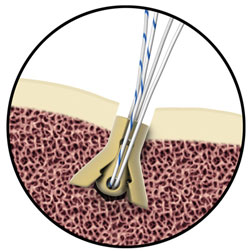 The Morphix® Suture Anchor is made of injection-molded Zeniva® PEEK for biocompatibility, radiolucent properties, and high strength. It offers improved cyclic loading stability which means less chance of the surgical repair failing during the healing process. The Morphix® Suture Anchor deploys dynamic wings with a high bearing area into the cancellous bone beneath the cortical shelf for improved device fixation.
The Morphix® Suture Anchor is made of injection-molded Zeniva® PEEK for biocompatibility, radiolucent properties, and high strength. It offers improved cyclic loading stability which means less chance of the surgical repair failing during the healing process. The Morphix® Suture Anchor deploys dynamic wings with a high bearing area into the cancellous bone beneath the cortical shelf for improved device fixation. The ExoShape® Soft Tissue Fastener is designed for fixation of the soft tissue graft on the tibial side of the knee joint in ACL reconstructions. It offers unparalleled accuracy to ensure the most anatomic and stable reconstruction, strong fixation, complete graft protection, simplified insertion, and total biocompatibility. The ExoShape® sheath is machined from 6-mm, 9-mm, and 13-mm Zeniva® PEEK rod.
The ExoShape® Soft Tissue Fastener is designed for fixation of the soft tissue graft on the tibial side of the knee joint in ACL reconstructions. It offers unparalleled accuracy to ensure the most anatomic and stable reconstruction, strong fixation, complete graft protection, simplified insertion, and total biocompatibility. The ExoShape® sheath is machined from 6-mm, 9-mm, and 13-mm Zeniva® PEEK rod.